Carbon monoxide is a ligand that forms
complexes with most transition metals, "metal carbonyls". Although carbon
monoxide is not a strong Lewis base, it does form
strong bonds to transition metals. The carbonyl group is much used in organometallic
syntheses because it forms metal carbonyls which are useful starting materials
for other organometallics. To consider why carbon monoxide forms such strong
bonds let's look at it structure and keybonding orbitals:
The grey orbitals are occupied and the blank one's
are empty. (Filled 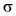 orbital within
orbital within 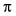 cloud is not shown)
cloud is not shown)
The carbon monoxide molecule is taken as bonding through a lone pair
of electrons located in an sp hybrid orbital on carbon. This filled *
antibonding molecular orbital can donate an electron to the metal atom
to form a sigma bond. The empty 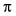 *
antibonding orbitals of the carbon monoxide are available to accept electrons
from suitable orbitals on the metal as shown below. Evidence for this comes
from measurements of bondlengths and bondorders.
*
antibonding orbitals of the carbon monoxide are available to accept electrons
from suitable orbitals on the metal as shown below. Evidence for this comes
from measurements of bondlengths and bondorders.
This animated picture shows the different steps of the C-O bonding proces.
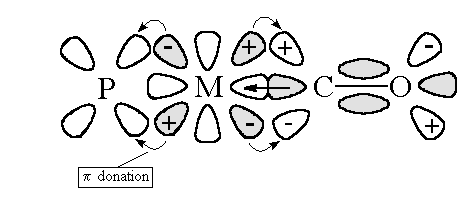
Normal  donation
of lone pairs on the ligand to empty orbital on the metal (usually one
of the tg eg: -dx2-y2)
Also
donation
of lone pairs on the ligand to empty orbital on the metal (usually one
of the tg eg: -dx2-y2)
Also 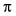 bonding
is possible between full orbitals on the metal (t2g eg: dxy,
tzy) to empty
bonding
is possible between full orbitals on the metal (t2g eg: dxy,
tzy) to empty 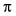 *
orbitals on the carbon monoxide. This
*
orbitals on the carbon monoxide. This 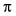 back bonding (also known as synergistic bonding) has two effects:
back bonding (also known as synergistic bonding) has two effects:
1. It strengthens the M-C bond.
2. The presence of extra electron density in 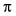 *
orbital on carbon monoxide weakens the C-O bond.
*
orbital on carbon monoxide weakens the C-O bond.
Carbon monoxide is an example of a 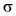 -donor
/
-donor
/ 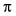 -acceptor ligand.
-acceptor ligand.
Here is an interesting
link about metal carbonyls.
To determine the structure of an (unidentified) substance,
infrared spectroscopy is much used because of it's ease of use and wide application.
In determining the structure of organometallics the carbonyl group is one
of the most important groups to analyse for.
Here are spectra of a monomer and a dimer
structure containing carbonyl groups. As you can see the terminal C-O bond
stretches at a higher frequency than the bridging one. Free carbon monoxide
has an even higher frequency (2143 cm-1)! Consider the following
equation:
E = h * 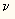
This means that the energy of a bond equals Planck's constant multiplied
by the frequency of its vibration (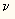 ).
Now we see that the C-O bond in the terminal carbonyl group is stronger
than the bond in the bridging group, how is that possible?
).
Now we see that the C-O bond in the terminal carbonyl group is stronger
than the bond in the bridging group, how is that possible?
Carbon monoxide gas has the strongest C-O bond. Decreased
C-O bond strengths (like in carbonyl groups) reflect increased electrondensity
in the 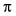 * orbitals.
This is due to the
* orbitals.
This is due to the 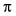 backbonding
from the metal, which increases the electron density in the
backbonding
from the metal, which increases the electron density in the 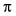 *
orbital of the CO ligand and weakens the C-O bond. Therefore, in a bridging
carbonyl ligand, there are two metal atoms donating electrons to the carbonyl's
*
orbital of the CO ligand and weakens the C-O bond. Therefore, in a bridging
carbonyl ligand, there are two metal atoms donating electrons to the carbonyl's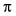 *
orbital weakening the bond even further.
*
orbital weakening the bond even further.
Even the kind of metal makes difference in the rate of 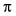 back bonding as a result of differences in diameter and electronic configuration.Besides
the kind of metal there are more factors who will have a big influence
on the C-O bond, like the nature of the ones attached on the metal. For
example (PCl3)FeCO3 is a monomer, the PCl3
group is an electron attracting group because it is a good
back bonding as a result of differences in diameter and electronic configuration.Besides
the kind of metal there are more factors who will have a big influence
on the C-O bond, like the nature of the ones attached on the metal. For
example (PCl3)FeCO3 is a monomer, the PCl3
group is an electron attracting group because it is a good 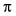 acceptor.
Other ligands can be
acceptor.
Other ligands can be 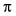 acceptor too which are all like PR3 (R= alkyl, aryl, halide).
These molecules are shaped like ammonia, eg:- PMe3, PPh3,
PPh2Et, PPh2Cl, PCl3. These molecules
have a lone pair of electrons in a sp3 hybrid orbital for -donation.
Unlike ammonia, PR3 has empty 3d-orbitals (P=3s23p3)
which can also accept electrons from the filled metal t2g orbitals.
Other examples of
acceptor too which are all like PR3 (R= alkyl, aryl, halide).
These molecules are shaped like ammonia, eg:- PMe3, PPh3,
PPh2Et, PPh2Cl, PCl3. These molecules
have a lone pair of electrons in a sp3 hybrid orbital for -donation.
Unlike ammonia, PR3 has empty 3d-orbitals (P=3s23p3)
which can also accept electrons from the filled metal t2g orbitals.
Other examples of 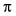 acceptors are
NO, N2, CR2.
acceptors are
NO, N2, CR2.
The PCl3 attracts electrons more strongly than the CO group.
As a result, the electron density in the carbonyls 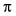 *
orbitals decreases and the C-O bond becomes stronger! Here are some typical
values.
*
orbitals decreases and the C-O bond becomes stronger! Here are some typical
values.
There are three different methods for
the preparation of metal carbonyls, the direct reaction, the reduction
reaction and thedisplacement reaction.
The Direct Reaction:
Here metal atoms react directly with the gaseous
carbon monoxide under certain conditions. This is the most simple reaction
but only works only for iron and nickel. Here's an
example.
The Reduction Reaction:
In this type of preparation the reaction starts
with a metal salt which, added to a ligand and a reducing agent, gives
the desired metal carbonyl. This reaction is also called a reductive carbonylation.
These reactions involve using very high pressures and temperatures. Here
are two examples:
The Displacement Reaction
The reaction starts with a metal carbonyl and
ends with a multi nuclear metal carbonyl. Here's
an example:
There are some typical metal carbonyl compounds
displayed in this table.
orbital within
cloud is not shown)
*
antibonding orbitals of the carbon monoxide are available to accept electrons
from suitable orbitals on the metal as shown below. Evidence for this comes
from measurements of bondlengths and bondorders.
donation
of lone pairs on the ligand to empty orbital on the metal (usually one
of the tg eg: -dx2-y2)
Also
bonding
is possible between full orbitals on the metal (t2g eg: dxy,
tzy) to empty
*
orbitals on the carbon monoxide. This
back bonding (also known as synergistic bonding) has two effects:
*
orbital on carbon monoxide weakens the C-O bond.
-donor
/
-acceptor ligand.
