

A compound that contains a metal-carbon bond. A metal is seen as any element less electronegative than carbon (this includes B, Si and As - and excludes P and halogen) top
The nomenclature for organometallics involves elements from classical organic and inorganic nomenclature with some extra rules. These rules mainly explain the structure of the compound and can indicate how the metal is bonded to the organic parts.
![]() <==
indicates number of carbons bonded to the metal.
<==
indicates number of carbons bonded to the metal.
Some examples
.
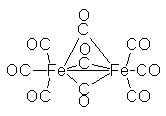 |
Here CO is acting as both a terminal and a bridging ligand. To indicate this in the name use the symbol "mu": µ.
![]() <== indicates
number of (metal)atoms being bridged.
<== indicates
number of (metal)atoms being bridged.
Some examples
NB: You can use nomenclature
at different levels for different purposes. At one end, eg:
- use simplest name Fe2(CO)9 ==> nonacarbonyldiiron
|
After you have studied the previous theory, you can practice some problems. top
Most ![]() -complexes
of the transition metals conform to an emperical rule, know as the "The
Eighteen Electron Rule ", which assumes that the metal atom accepts sufficient
electrons from the ligands to enable it to attain the electronic configuration
of the next noble gas. This means that the valence shell of the metal atom
contains 18 electrons. Thus, the sum of the of the number of metal d-electrons
plus the electrons conventionally regarded as being supplied by the ligands
is 18. This leads to a constant electronic arrangement for the metal atom.
-complexes
of the transition metals conform to an emperical rule, know as the "The
Eighteen Electron Rule ", which assumes that the metal atom accepts sufficient
electrons from the ligands to enable it to attain the electronic configuration
of the next noble gas. This means that the valence shell of the metal atom
contains 18 electrons. Thus, the sum of the of the number of metal d-electrons
plus the electrons conventionally regarded as being supplied by the ligands
is 18. This leads to a constant electronic arrangement for the metal atom.
(Reference: 3)
Procedure for counting electrons
Fe0 ==> 8 (e-) (4s2 3d6 or just count the number of groups in the "periodic system" example).
[Fe(L)x] 0 ==> 8 (e-)
[Fe(L)y] +==> 7 (e-)
[Fe(L)z] - ==> 9 (e-)
terminal CO ==> 2 (e-)
bridging CO ==> 1 (e-)
metal-metal bond ==> 1 (e-)
organic ligands ==> number of electons depends on the group: see
table
Some examples of the Eighteen Electron Rule
(Click on the structures to see the ligand classification
table)
Fe(CO)5:
Fe2(CO)9:
In this case the molecule is split in half because of the two iron atoms which
both want to attain the electronic configuration of the next noble gas.
eg: Mn(CO)5
This molecule is unstable, here are the two ways to fill the valence shell of the mangenese:
Dimerisation:
Accepting an electron:
The Eighteen Electron Rule will indicate stoichiometries and
possible geometries of compounds. This rule will not differentiate between
possible structures, to determen the actual structure of a compound techniques
like IR or NMR are necessary.
Here's an example:
Bis[tetracarbonylcobalt] can exist in two forms:
| Structure A: |
Coo: 9e- 4xCOT: 8e- Co-Co bond: 1e- Total: 18e- |
| Structure B: |
Coo: 9e- 3xCOT: 6e- 2xCOB: 2e- Co-Co bond: 1e- Total: 18e- |
Structure A as well as structure B are possible. From NMR spectra it appears that structure A is the solid state and low temperature-solution state and structure B is the high temperature-solution form.
To fill the outer shell with 18 electrons there must be 18 electrons available for bonding.
After you have studied the previous theory, you can practice some problems. top